Research Article Download PDF
Immunological Study of Arsenic Exposed Population
Received: May 17, 2019; Published: June 04, 2019
Abstract
Arsenic poisoning through ground water is causing lot of health hazards in the population of Bihar. Therefore, the present study aims to assess the immunological parameters in blood samples of the village people of arsenic hit areas of Bhojpur district. The study was conducted on 100 volunteers of arsenic hit area of Bhojpur district of Bihar.The present study shows that arsenic does not cause any adverse effecton total leukocyte count however arsenic exposure increased the TLC level in few people. The neutrophil, eosinophil, basophil and monocyte (%) of blood were found to be normal. Hence, the study concludes that arsenic mildly causes damage to the immunity of the population of arsenic hit area of Bhojpur district.
Keywords: Arsenic; White Blood Cells; Human Subjects; Bhojpur district
Introduction
In India investigations carried out by Central Ground Water Board (CGWB) reveals that arsenic contamination (>0.05 mg/L) is affecting the states of West Bengal, Bihar, Uttar Pradesh, Assam, Chhattisgarh. The Bengal Delta Plain (BDP) covering Bangladesh andWest Bengal in India is the most severe case of groundwater arsenic contamination. Besides this high Arsenic ground water has also been reported from Jharkhand and Manipur state.In Bihar, arsenic was initially detected in the year 2002 from Semariya-Ojhapatti villages of Bhojpur district. The 57 blocks out of 18 districts are under high arsenic contamination risk in Bihar. Arsenic groundwater contamination in Bihar was initially detected in the year 2002 from Semariya-Ojhapatti villages of Bhojpur district. Detailed investigations in the Gangetic Plain of Bihar revealed its wide occurrence on both the banks of the river Ganga [1-4].
Arsenic exerts pleiotropic effects on immune cells, which may initiallyresult in the suppression of major immune functions. Notably, arsenic decreases bacterial phagocytosis by macrophages, reduces T cell proliferation and markedly represses the secretion of pro-inflammatory cytokines by activated DC and T cells. These inhibitions can increase the susceptibility to infection by limiting pathogen eradication. Arsenic-induced immune suppression likely increases the incidence of lower respiratory tract infections and diarrhea, which frequently develop in young exposed children from low-income countries [5]. Particularly, increased arsenic exposure was found to promote pulmonary tuberculosis in Chile [6] and visceral leishmanios is in India [7]. In a murine model, chronic exposure to low doses of arsenic also increased morbidity of influenza A infection by repressing DC-mediated immune responses in mice [8].
Arsenic has been reported to be responsible for defective cell mediated immunity and decreased percentage of T-helper cells in the body [9]. Immunosuppressive action of arsenic has been responsible for decreased spleenicphagocytic activity of macrophages [10]. Arsenic administration results in delayed type hypersensitivity decrease in MCV of RBC and decrease in the weight oforgans like thymus and spleen which are the active sites for anti body production [11]. Decreased immunity makes the animals susceptible to diseases making them unthrifty and uneconomic. There are also altered haematological parameters in case of chronic exposure to arsenic. Some reports suggest an increase in the fragility of erythrocyte membrane by chronic arsenic administration. Arsenic toxicity is also held responsible for reduced growth and infertility in animals. Carcinogenicity has been reported because of arsenic toxicity in animals. The accumulating nature of arsenic in animal tissues as well as its secretion in milk may pose great threats to animals and human beings. As is also responsible for altered liver functionby binding with the thiol group of liver enzymes and some carrier proteins [12]thus, chronic arsenic exposure leads to various adverse effects on health, production and reproduction in animals.
Materials and Methods
Ethical Approval: The ethical approval was obtained from the Post Graduate Research Council (PGRC) of the Veer Kunwar Singh University, Ara, Bhojpur, Bihar.
Collection of Blood: The blood samples from the peripheral venous were drawn from the 100 volunteers (51 male & 49 female) of the village from arsenic hit area of Bhojpur district. The blood samples were collected in EDTA vaccutainer and were labelled and stored at 4 0C for haematological study.
Haematological Evaluation: The haematological parameters Total Leukocyte Count (TLC), Differential Leukocyte Counts (DLC) like –Neutrophils, Lymphocytes, Monocytes, Eosinophils, Basophilswere counted as per the protocols.
Statistical Analysis: Results are presented as mean ± SD and total variation present in a set of data was analysed through one way analysis of variance (ANOVA). Difference among mean values has been analysed by applying Dunnet’s t-test. Calculations were performed with the Graph Pad Prism Program (Graph Pad software, Inc., San Diego, U.S.A.). The criterion for statistical significance was set at P< 0.05.
Results
Total leukocyte Count(TLC): The TLC were observed less than 11000 in 84 subjects and in16 subjects were observedmore than 11,000 (Figure 1).
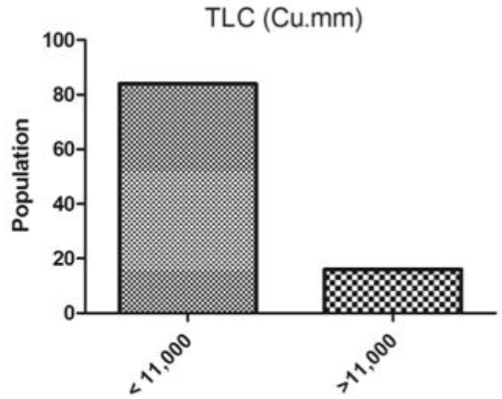
Figure 1: TLC counts of subjects of arsenic hit area of Bhojpur district (ANOVA-Dunnett’s Test, P<0.05).
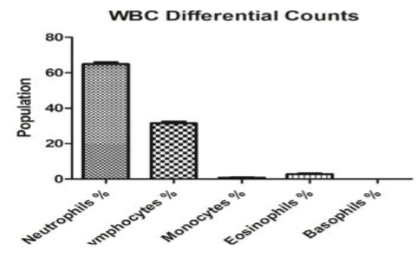
Differential Leukocyte Count (DLC): Under differential leukocyte count, all the granulocytes and agranulocytes were studied. During the study, the mean lymphocyte content (%) of blood was found to be 35.37±1.04. The neutrophil, eosinophil, basophil and monocyte (%) of blood were found to be 65.77± 0.60 and 5.74± 0.64; 0.01± 0.03 and 0.45± 0.05; respectively (Figure 2).
Discussion
Arsenic has been found to induce apoptosis in various cell systems. This condition leads to a defective cell-mediated immunity and decreased percentage of T cell and T helper cell subpopulations in peripheral mononuclear cells thus adversely affecting the T cell survival and function in mononuclear cells [9]. Anarsenic concentration higher than 1μM induces release of tumour necrosis factor from the mononuclear cells and causes cytotoxic effect on T cells. Spleenic macrophages are the essential sites for trapping and concentrating foreign substances carried in the blood and for synthesis and release of antibodies to the circulation. In vivo exposure of rodents to sodium arsenite (0.5 mg/Kg bodyweight) decreases the phagocytic activity for ingestion and digestion of exogenousantigens such as whole microorganisms, as evident from the phagocytic index,11444.55 ± 62.86 (in control) to 5555.5 ± 1571.33 in arsenic treated rates. Decreased phagocytic index indicates higher susceptibility of animals to diseases. Effect of inorganic arsenicals on DNA synthesis in unsensitised human bloodly mphocytes is biphasic. These chemicals at very low concentrations enhance blast formation and DNA synthesis. Maximum stimulating effect is observed at the concentration of 1 x 10-5M to 2 x 10-6M for sodium arsenite exposure whereas for sodium arsenate, the concentration varies form 1 x 10-5 M to 1 x 10-6M [13]. The longer the exposure of the lymphocytes to arsenicals, the lower the concentrations of arsenicals at which the maximum stimulating effect is found. It isobserved that the stimulating effect of trivalent arsenic (sodium arsenite) is strongerthan pentavalent arsenic (sodium arsenate). By inhibiting the DNA synthesis oflymphocytes, arsenic affects the cellular immunity adversely.Supplementation of arsenic through drinking water has been found tosuppress the natural, humoral and cell mediated immune response in broiler chicks [14]. A deficiency of dietary vitamins and minerals causes increased sensitivity to the adverse effects of arsenic contaminated drinking water.
The present study observed high TLC in 16 people (>11,000) and normal value in 84 people. The neutrophil, eosinophil, basophil and monocyte (%) of blood were found to be normal [15]reported that monocyte-derived macrophages from arsenic-exposed individuals with skin lesions exhibited reduced cell adhesion capacity, F-actin expression, nitric oxide production and phagocytic activity. In vitro, 0.5–2 μM arsenic trioxide (ATO) was shown to block the differentiation of human peripheral blood monocytes into functionalmacrophages by repressing NF-κB-related survival pathways [16].
Short treatments of human mature macrophages with non-cytotoxic arsenic concentrations (1–5 μM, 6 h) was shown to inhibit the transcriptional activity of the liver X receptor, resulting in the decreased expression of its target genes, ABCA1 and SREBP-1c, and impaired cholesterol efflux [17]Prolonged treatment (3–6 days) with ATO was shown to de-differentiatemacrophages into CD14-expressing monocytic- like cells, notably through the reorganization of the actincytoskeleton [18].
Thus, present study shows that arsenic contamination in the present arsenic hit area population ofBhojpur district, do not show significant immunological changes, however one fourth percentage of the population are at the risk of getting the disease.
Conclusion
The present study was conducted on 100 volunteers of arsenic hit area to evaluate the effect of arsenic exposure on immunological parameter. Thus, in the light of present investigation the study concludes that arsenic did not have any serious adverse effect on total leukocyte count, however arsenic exposure increased the TLC level in few percentof the population.
References
- Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, et al. (2016) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152: 520-529.
- Chakraborti D, Rahman MM, Chowdhury UK, Paul K, Chowdhury UK, et al. (2002) Arsenic calamity in the Indian subcontinent: What lessons have been learned?. Talanta 58: 3-22.
- Bhattacharya P, Welch AH, Stollenwerk K G, McLaughlin MJ, Bundschuh J (2007) Arsenic in the environment: Biology and Chemistry. Science of the Total Environment 379: 109-120.
- Hong TZ (2017) Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Current Science 97(6): 753- 755.
- Rahman M, Vahter EC, Ekström, LA Persson (2011) Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect 119: 719-724.
- AH Smith, G Marshall, Y Yuan, J Liaw, Ferreccio C, et al. (2011) Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol 173: 414-420.
- Hong TZ (2017) Meghan R Perry, Vijay K Prajapati, Joris Menten, Andrea Raab, Joerg Feldmann, et al. (2015) Arsenic exposure and outcomes of antimonial treatment in visceral leishmaniasis patients in Bihar, India: a retrospective cohort study. PLoSNegl Trop Dis 9.
- Kozul KH Ely, RI Enelow, JW Hamilton (2009) Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect 117: 1441-1447.
- Yu HS, Liao WT, Chang KL, Yu GL, Chen GS (2002) Arsenic induces tumor necrosis factor alpha release and tumor necrosis factor receptor 1signalling in T helper cell apoptosis. J Invest Dermatol 119(4): 812-819.
- Sengupta M, Bishayi B (2002) Effect of lead and arsenic on murine macrophage response. Drug Chem Toxicol 25(4): 459-472.
- Schulz H, Nagymajtenyi L, Institoris L, Papp A, Siroki O (2002) A study on behavioral, neurotoxicological and immunotoxicological effects of subchronic arsenic treatment in rats. J Toxicol Environ Health 5(16): 1181- 1193.
- Meng ZQ, Meng NY (2000) Effects of arsenic on blast transformation and DNA synthesis of human blood lymphocytes. Chemosphere 41(1-2): 115-19.
- Vodela JK, Lenz SD, Renden JA, McElhenney WH, Kemppainen BW (1997) Drinking water contaminants (arsenic, cadmium, lead, benzene, and trichloroethylene). Effects on reproductive performance, egg quality, and embryo toxicity in broiler breeders. Poult Sci 76(11): 1493- 1500.
- Banerjee S, Banerjee R, Sen A, Bandyopadhyay N, Sarma P, et al. (2009) Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J ClinImmunol 29: 582-594.
- Lemarie A, C Morzadec, D Mérino, O Micheau, O Fardel, et al. (2006) Arsenic trioxide induces apoptosis of human monocytes during macrophagic differentiation through nuclear factor-kappaB-related survival pathway down- regulation. J Pharmacol Exp Ther 316(1): 304-314.
- Padovani AM, Molina MF, KK Mann (2010) Inhibition of liver x receptor/retinoid X receptor-mediated transcription contributes to the proatherogenic effects of arsenic in macrophages in vitro. Arterioscler Thromb Vasc Biol 30: 1228-1236.
- Lemarie, E Bourdonnay, C Morzadec, O Fardel, L Vernhet (2008) Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway. J Immunol 180: 6010-6017.
- 19. Vodela JK, Lenz SD, Renden JA, McElhenney WH, Kemppainen BW (1997) Drinking water contaminants: Effects on reproductive performance, egg quality and embryo toxicity in broiler breeders, Poultry Science 76(11): 1493-1500.