Mini Review Download PDF
Cancer Immunotherapy: Why, What Has Been Achieved and Future Directions
Received: July 01, 2019; Published: July 08, 2019
Abbreviations
CAR: Chimeric Antigen Receptor; TAA: Tumor Associated Antigens; DC: Dendritic Cells; APC: Antigen-Presenting Cells; AML: Acute Myeloid Leukemia; TME: Tumor Micro-Environment; CIC: Cancer Initiating Cells; ETC: Electron Transport Chain
Mini Review
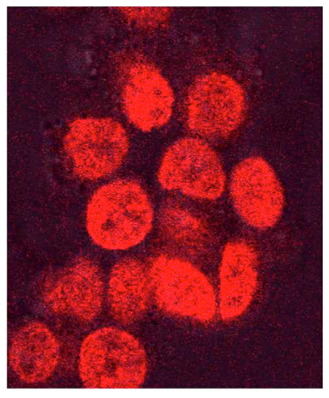
Every sixth death in the world is due to cancer, making it the second leading cause of death (second only to cardiovascular diseases) [1]. In 2017, 9.6 million people are estimated to have died from the various forms of cancer. According to National Cancer Institute (NIH; https://www.cancer.gov/about-cancer/understanding/statistics), in the US, based on data from 2011 to 2015, the number of new cases of cancer (cancer incidence) is 439.2 per 100,000 men and women per year and the number of cancer deaths (cancer mortality) is 163.5 per 100,000 men and women per year. According to International Agency for Research on Cancer (IARC; French: Centre International de Recherche sur le Cancer, CIRC), WHO (http://www-dep.iarc.fr/WHOdb/WHOdb.htm), and Globocan statistic (https://www.wcrf.org/dietandcancer/cancer-trends/data-cancer-frequency-country), several European and Asian countries have huge cancer burden. With increasing life expectancy, the deaths due to cancer is on rise [1]. Once the number of deaths is corrected for population size, it is observed that cancer death rates have approximately flat-lined; then when further corrected for age it is observed that globally, death rates are falling [1]. This represents progress, although very slow. The progress is slow despite huge advances in surgery, chemotherapy, improved radiotherapy [2], better early detections and vastly improved disease management [3]. A better understanding of the immune system has contributed to these improvements [4]. However, emergence of drug resistance or resistance toward radiation, occult cancers and residual disease are perhaps the major challenges. One factor that has been long overlooked is the global human cytomegalovirus (HCMV) burden [5] and its relation to cancer [6]. Most works till date tried to focus on oncogenic potential of HCMV [7], and studies on oncomodulation [8] of HCMV mostly are restricted to cancer progression [9-10]. Interestingly we (Mookerjee A and Davrinche C; unpublished) have observed that HCMV infection in vitro can actually impart cancer cells resistance to chemotherapeutic drugs (Figure 1). This might imply that HCMV prefers immunosuppressed environment of the tumor and might further imply that cancer patients who are positive for HCMV might quickly develop multi-drug resistance (MDR). These challenges of drug resistance and residual disease are perhaps best dealt with through immunotherapy and immune-chemo-therapy.
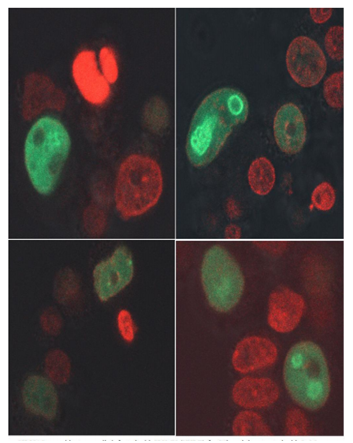
SK-NAS neuroblastoma cells infected with HCMV (VHLE) for 96h and then treated with 2µM doxrubicin for 3h
Different immunotherapies for cancers are now available with different levels of success. Immunotherapy involving engineered T cells have met limited, but definite success. T cells are engineered to express a chimeric antigen receptor (CAR) against a tumor antigen. CAR-T, especially targeting CD19 has reshaped how hematological malignancies are treated. Despite the overwhelming early clinical success, CAR-T therapies are associated with severe side-effects, disease relapse, often exhibit limited efficacy, especially due to our limited knowledge about tumor associated antigens (TAA) that can be used as targets. Now, this immunotherapy is being further improved [11].Beside CAR-T, adoptive transfer of T-cells is being tried with limited success. Patient’s γδ T-cells are expanded ex-vivo in presence of pyrophosphates and then re-introduced [12]. Although this therapy showed some promise in animal models and laboratory conditions [13-15], the clinical efficacy has not been very encouraging so far [12].
The utility of αβ T-cells in cancer immunotherapy has remained stunted due to a lack of knowledge about TAA. Use of NY-ESO1 [16] as the TAA yielded limited success due to easy immune-evasion. Now serious efforts are being made to identify tumor reactive T cells and to expand them for immunotherapy [17].Another approach toward immunotherapy is to use dendritic cells (DC). DC vaccination offers a lot of promise due to the fact that these antigen-presenting cells (APC) can cross-present antigens and activate both CD4 and CD8 T-cells. Some success has been achieved in the clinic, especially for Acute Myeloid Leukemia (AML) [18]. However, DC vaccination begs serious improvements [19]. Recently, a lot of work has been done in animal models to improve DC vaccines [20-21].
However, beside lack of our knowledge about TAA, immunotherapy also suffers hindrances from immune checkpoints. These naturally occurring check-points that are meant to put brakes on an on-going immune response when it is no longer needed to protect our body, prevent auto-immunity and allow generation of memory T-cells [22] are over-expressed during tumor progression to stunt the immune system and hinder immunotherapeutic efforts. Some success has been achieved by blocking the PD1-PDL1 immune checkpoint, especially in case of melanoma, in the clinic. After successful clinical trial (NCT01866319) Keytruda (Pembrolizumab) a humanized anti-PD1 antibody is already in the clinic and has yielded some great results. It is now being tested for lung cancer [23]. Other brands of anti-PD1 are also in the clinic (Nivolumab and Cemiplimab). Also, anti-PDL1 antibodies are available (Atezolizumab, Avelumab and Durvalumab) that are used for treating some cancers like bladder cancer. Interestingly, Patients who receive anti-PD-1 have an average response rate of 30 percent but approximately 25 percent of the patients experience tumor relapse within two years after treatment has stopped. Considerable success with longer duration has been using anti-CTLA4 antibody, Yervoy (Ipilimumab) in melanoma [24] and led to Dr. James P. Allison winning the Noble Prize in Physiology and Medicine in 2018. Although anti-CTLA4 has serious side-effects, for otherwise untreatable late stage cancers, a combination of Ipilimumab and Nivolumab is being tested [25]. Beside these, efforts are ongoing to target adenosine axis and other immune checkpoints [26] and develop next-generation pattern recognition agonists and nano-medicine to target tumor micro-environment (TME) [27,28].
Beside checkpoint inhibitors and targeting TME, other approaches are being extensively tested in laboratories. One approach is to understand the biology of cancer stem cells or Cancer Initiating Cells (CIC) [29]. Another approach is to understand the metabolic pathways of the immune cells to prolong immune reaction against tumor cells. It is thought that often immune cells are not metabolically capable of mounting an immune response and immunotherapies fail as it tries to push these metabolically incapable cells toward mounting an immune response, which is almost like whipping a lame horse to win the race. It has been seen that the serum copper level rises in cancer patients [30]. It appears that the copper stores in the body including liver and immune cells are drained and the copper enters serum. This not only helps in angiogenesis [31,32] but also causes immunesuppression. The immune cells, especially the T-cells are forced into glycolytic pathway and can only follow effector function that leads to exhaustion [33,34]. This not only occurs due to dying tumor cells releasing potassium [35] but also perhaps due to mitochondrial dysfunction due to lack of copper. A mitochondrial dysfunction would restrict the Electron Transport Chain (ETC) and force glycolytic pathway only [36]. Since the immune system needs copper to function properly [37] studies are being conducted in animal models on introducing copper as copper chelates (copper co-ordinate complex) in minute doses to restore proper immune function [38-40].
References
- Naghavi M (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016.Lancet390(10100):1151-1210.
- Chen HHW, Kuo MT (2017) Improving radiotherapy in cancer treatment: Promises and challenges.Oncotarget 8(37): 62742-62758.
- Sheetz KH, Justin BD, Hari N (2019) Centralization of High-Risk Cancer Surgery Within Existing Hospital Systems. J clin Oncol.
- Yazaki P, Thinzar ML, Megan M, Lin Li, Anakim S, et al. (2019) Improved antibody-guided surgery with a near-infrared dye on a pegylated linker for CEA-positive tumors. J Biomed Opt 24(6): 1-9.
- Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK (2013) The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev 26(1): 86-102.
- Michaelis M. et al. (2009) The Story of Human Cytomegalovirus and Cancer: Increasing Evidence and Open Questions. Neoplasia 11(1): 1-9.
- Herbein G, Kumar A (2014) The Oncogenic Potential of Human Cytomegalovirus and Breast Cancer. Front Oncol4: 230.
- Herbein G (2018) The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 10(8): 408.
- Pasquereau S, F Al Moussawi, W Karam, M Diab Assaf, A Kumar, et al. (2017) Cytomegalovirus, Macrophages and Breast Cancer. Open Virol 11:15-27.
- Johnsen JI, N Baryawno, C S Naucler (2011) Is human cytomegalovirus a target in cancer therapy? Oncotarget 2: 1329-1338.
- Panagopoulou TI, Rafiq QA (2019) CAR-T immunotherapies: Biotechnological strategies to improve safety, efficacy and clinical outcome through CAR engineering. Biotechnol Adv.
- Legut M, Cole DK, Sewell AK (2015) The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell Mol Immunol 12(6): 656-668.
- Mookerjee-Basu J, Vantourout P, Martinez LO, Perret B, Collet X, et al. (2010) F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol 184(12):6920-6928.
- Vantourout P, Rolland C, Pont F, Martin H, Davrinche C, et al. (2009) Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol 183(6): 3848-3857.
- Daguzan C, Kulyk-Barbier H, Davrinche C, Peyrottes S, Champagne E, et al. (2016) Aminobisphosphonates Synergize with Human Cytomegalovirus To Activate the Antiviral Activity of Vγ9Vδ2 Cells. J Immunol 196(5): 2219-2229.
- 16. Odunsi K (2017)Immunotherapy in ovarian cancer. Ann Oncol 28(suppl_8):viii1-viii7.
- Costa-Nunes C, Amélie Cachot, Sara Bobisse, Marion Arnaud, Raphael Genolet, et al. (2019) High-throughput Screening of Human Tumor Antigen-specific CD4 T Cells, Including Neoantigen-reactive T Cells. Clin Cancer Res.
- Brien LJ, Guillerey C, Radford KJ (2019) Can Dendritic Cell Vaccination Prevent Leukemia Relapse? Cancers (Basel) 11(6): 875.
- Sabado RL, Sreekumar B, Nina B (2017) Dendritic cell-based immunotherapy. Cell Res 27(1): 74-95.
- Mookerjee A, Michele G, Lana EK (2018) A cancer vaccine with dendritic cells differentiated with GM-CSF and IFNα and pulsed with a squaric acid treated cell lysate improves T cell priming and tumor growth control in a mouse model. Bioimpacts 8(3): 211-221.
- Mookerjee A, Michele G, Lana EK (2019) IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine. Cancers (Basel) 11(1): 40.
- Odorizzi PM, Kristen E Pauken, Michael A Paley, Arlene Sharpe, E John Wherry (2015) Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med212(7): 1125-1137.
- Gandhi L, Delvys RA, Shirish G, Emilio E, Flavia DA, et al. (2018) Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N Engl J Med 378: 2078-2092.
- 24. Couzin-Frankel J (2013) Cancer Immunotherapy.Science 342(6165): 1432-1433.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, et al. (2013) Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med 369: 122-133.
- Vigano S, Dimitrios A, Melita I, Christine M, Christophe C, et al. (2019) Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front Immunol 10: 925.
- ODonovan DH, Yumeng Mao, Deanna A Mele (2019) The Next Generation of Pattern Recognition Receptor Agonists: Improving Response Rates in Cancer Immunotherapy. Curr Med Chem.
- Zanganeh S, Spitler R, Hutter G, Ho JQ, Pauliah M, et al. (2017) Tumor-associated macrophages, nanomedicine and imaging: the axis of success in the future of cancer immunotherapy. Immunotherapy 9(10): 819-835.
- Maccalli C, Parmiani G, Ferrone S (2017) Immunomodulating and Immunoresistance Properties of Cancer-Initiating Cells: Implications for the Clinical Success of Immunotherapy. Immunol Invest 46(3): 221-238.
- Zowczak M, Iskra M, Torliński L, Cofta S (2001) Analysis of serum copper and zinc concentrations in cancer patients. Biol Trace Elem Res 82(1-3):1-8.
- Urso E and Maffia M (2015) Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J Vasc Res 52(3): 172-96.
- Hendriksen EM, Span PN, Schuuring J, Peters JP, Sweep FC, et al.(2009) Angiogenesis, hypoxia and VEGF expression during tumour growth in a human xenograft tumour model. Microvasc Res 77(2): 96-103.
- Buck MD, Sowell RT, Kaech SM, Pearce EL (2017) Metabolic Instruction of Immunity. Cell 169(4): 570-586.
- Pearce EJ and Pearce EL (2018) Immunometabolism in 2017: Driving immunity: all roads lead to metabolism. Nat Rev Immunol 18(2): 81-82.
- Baixauli F, Villa M, Pearce EL (2019) Potassium shapes antitumor immunity. Science 363(6434): 1395-1396.
- Klein Geltink RI, Lambrecht BN, Rambold AS, Pearce EJ, Pearce EL, et al. (2017) Mitochondrial Priming by CD28. Cell 171(2): 385-97.
- 37. Percival SS (1998) Copper and immunity. Am J Clin Nutr 67(5 Suppl):1064S-1068S.
- Mookerjee A, Mookerjee Basu J, Dutta P, Majumder S, Bhattacharyya S, et al. (2006) Overcoming drug-resistant cancer by a newly developed copper chelate through host-protective cytokine-mediated apoptosis. Clin Cancer Res 12(14 Pt 1):4339-4349.
- Chatterjee S, Ananda M, Jayati M, paramita C, Avishek G, et al. (2009) A novel copper chelate modulates tumor associated macrophages to promote anti-tumor response of T cells. Plos One 4(9):e7048.
- Chakraborty P, Chatterjee S, Ganguly A, Saha P, Adhikary A, et al. (2012) Reprogramming of TAM toward proimmunogenic type through regulation of MAP kinases using a redox-active copper chelate. J LeukocBiol 91(4):609-619.