Review Article Download PDF
Use of Continuous Glucose Monitoring in Neonates
Received: May 16, 2019; Published: June 05, 2019
Abstract
Neonatal hypoglycemia remains one of the most common controversial topics in the field of neonatology. Use of Continues glucose monitoring (CGM) in the adult population is well established. In this review, we present the recent updates about the use of CGM devices in both preterm and term neonates.
Keywords: Hypoglycemia, CGM, Neonates, Glucose
Abbreviations CGM: Continuous Glucose Monitoring; BG: Blood Glucose; MARD: Mean Absolute Relative Difference; IVH: Intraventricular Hemorrhage
Use of Continuous Glucose Monitoring in Neonates
Glucose monitoring is an essential part of the management of glucose-related diseases such as diabetes and neonatal hypogly-cemia. Throughout the time (figure 1), there has accelerated technological advancement of CGM devices and techniques, to provide more accurate, quality of life supportive management for lifelong disease, to help guide the clinicians as well patients in the management of the related disorders [1]. Continuous glucose monitoring CGM device can be used to follow glucoselevels twentyfour hours [2].The sensor measures interstitial glucose level. These devices typically consist of three parts: A temporary needle sensormeasures the interstitialglucoselevelsconnected to a transmitter by the different interface based on the manufacture, and a receiver that record the data received by the sensor [3]. The use of CGM devices has the advantages of having real-time data about the body reaction to insulin, food, and other factors[4]. Also, it allows the chance to monitor blood glucose (BG) levels during less convenient times for patients (Overnight, prolonged exercise, fasting, and others) [5,6].Advancement in technology and research continuetoimprove the accuracy, feasibility, and availability of thesedevices for diabetic patients [7-9].The accuracy of CGM isbeing measured by the mean absolute relativedifference(MARD), which is the mean of the absolute differencesbetwe-en CGM and simultaneous reference values as a percentage of the reference value. Errors of ≤13% are generallyconsideredacceptable [10].
Although the rule of CGM is well established in thecare process for diabetes mellitus, its’ rule in neonatal glucose monitoring is less clear. Driven by the high incidence of neonatal hypoglycemia and the associated adverse neuro-developmental outcomes associated with it [11-13],multiple recent studies have looked into the use of CGM in newborns and preterm neonates. The challenge with the use of CGM devices inneonates arises from the relatively small size, limited subcutaneous fat, and the unclear understanding of the normal glucose hemostasis in neonates. Beardsall et al. [14]showed in their cohort of very low birth weight infants that the use of CGM devices in very low birth weight infants was safe and practical with very good correlation with the routine blood glucose measurements. This finding was later confirmed in more recent studies for the same populations [15-17].More recent technological advances in CGM unlinkedsensors target-ing preterm population resulted in more convenient nursing care and was associated with less procedural pain when com-pared to the regular blood glucose measurements methods [18,19].Use of CGM was also studied in infants at risk for hypoglycemia; Harris, Deborah L, et al. [20]followed 102 infants ≥ 32 weeks glucose levels with CGM devices, in themeantime in those infants, intermittentbloodglucosemeasure-ment using glucose oxidase test was performed. Theyfound that the CGM use resulted in significantly more episodesof hypoglycemia about 81% that were not detected by regular BG measurements. Another small sample study looked at thefeasi-bility of using CGM in term and near-term infants born tomother with diabetes [21],The authors concluded that the use of CGM is beneficial early after birth in this subset of infants.
Use of CGM to Predict Short and Long-termNeuro-developmental Outcomes
As the operational threshold and the BG levels that require interventions or result in adverse neurodevelopmentaloutcome-remains one of the most controversial questions in the field of the neonatology. Attempts have been made to use CGM data to compare longterm outcomes,McKinlay et al.[22]in largesample studyevaluated the neurological outcomes at two years of age between the infant with low BG< 47 mg/dl whoreceivedtreat-ment and those who had similar levelsdetected by the CGM, but didn’t receive treatment. No difference was found between the two groups regarding neurosensory impairment and processing difficulties. The authorsconcluded with the assist of themasked data obtained by the CGM that low BG levels<47werenot associated with adverseneurodevelopmental outcomes. Another small sample study to evaluate the short-term adverse outcomes suggested that the use of CGM can help detect IVH, as suggested by the glucose variability captured early by theuse of CGM devices [23].
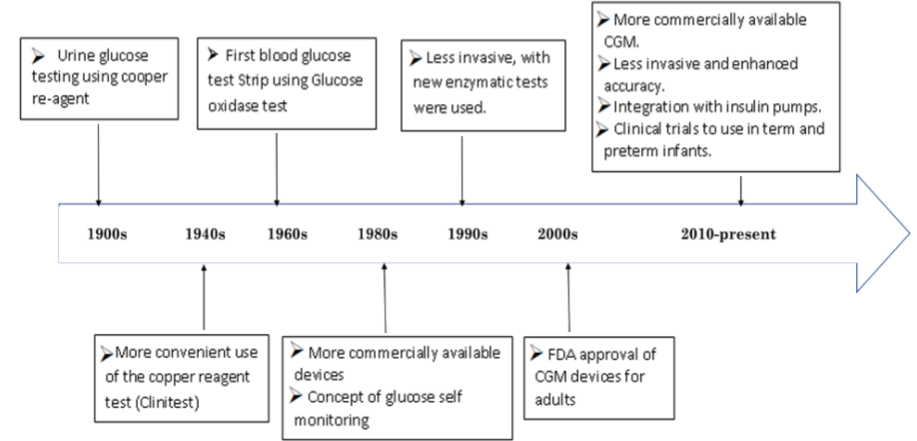
Figure 1: Timeline of glucose monitoring, FDA: Food and Drug Administration; CGM: Continuos Glucose Mounitoring.
Conclusion
CGM sensor use in term and preterm neonates is practical, safe and feasible. It does provide the advantages of real-time data, as well as decrease the babies discomfort by decreasing the frequency of blood sampling. Data without interventionobtain-ed by CGM didn’t show significant adverse outcomes as a result of neonatal hypoglycemia. Future research is needed to evaluate long term outcomes based on the interventions made according to the data obtained by CGM before adopting this new technology.
References
- Clarke S, Foster J (2012) A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. BrJ Biomed sci 69: 83-93.
- McKinlay CJ, Chase JG, Dickson J, Harris DL, Alsweiler JM, et al. (2017) Continuous glucose monitoring in neonates: A review. Maternal health, neonatology and perinatology 3: 18.
- Koschinsky T, Heinemann L (2001) Sensors for glucose monitoring: Technical and clinical aspects. Diabetes Metab Res Rev 17: 113-123.
- Group JDRFCGMS (2009) The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes care. 2009;32(8):1378-1383.
- Chase HP, Kim LM, Owen SL (2001) Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics 107: 222-226.
- Afandi B, Hassanein M, Roubi S, Nagelkerke N (2019) The value of Continuous Glucose Monitoring and Self-Monitoring of Blood Glucose in patients with Gestational Diabetes Mellitus during Ramadan Fasting. Diabetes Res Clin Pract 151: 260-264.
- Mastrototaro JJ, Cooper KW, Soundararajan G, Sanders JB, Shah RV (2006) Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Advances in therapy 23: 725-732.
- Yang Y, Gao W (2019) Wearable and flexible electronics for continuous molecular monitoring. Chemical Society Reviews.
- Tapsak MA, Rhodes RK, Shults MC, McClure JD (2019) Techniques to improve polyurethane membranes for implantable glucose sensors. In: Google Patents.
- Kasic VS, Pulichino AM, Gueydan M, Barlier A, David M, et al. (2004) A neonatal form of isolated ACTH deficiency frequently associated with Tpit gene mutations. Endocr Res 30: 943-944.
- Adamkin DH (2010) Clinical report-postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 127: 575-579.
- Cornblath M, Hawdon JM, Williams AF, Green AA, Platt MPP, et al., (2000) Controversies regarding definition of neonatal hypoglycemia: Suggested operational thresholds. Pediatrics 105:1141-1145.
- Gandhi K (2017) Approach to hypoglycemia in infants and children. Transl Pediatr6:408.
- Beardsall K, Vanhaesebrouck S, Stuart OA, Vanhole C, Van Weissenbruch M, et al., (2013) Validation of the continuous glucose monitoring sensor in preterm infants. Archives of Disease in Childhood-Fetal and Neonatal Edition. 98: F136-F140.
- Lluch M, Alminana N, Palomo A, Sanz M, Vidal X, et al., (2009) Continuous glucose monitoring in infants of very low birth weight. Neonatology 95: 217-223.
- Perri A, Giordano L, Corsello M, Priolo F, Vento Get, et al., (2018) Continuous glucose monitoring (CGM) in very low birth weight newborns needing parenteral nutrition: validation and glycemic percentiles. Ital J Pediatr 44:99.
- Galderisi A, Facchinetti A, Steil GM, Ortiz-Rubio P, Cavallin F, et al., (2017) Continuous glucose monitoring in very preterm infants: A randomized controlled trial. Pediatric 140: 2017-2162.
- Galderisi A, Lago P, Steil GM, Ghirardo M, Cobelli C, et al., (2018) Procedural pain during insertion of a continuous glucose monitoring device in preterm infants. J Pediatr 261-264.
- Zecca E, Tiberi E, Perri A, Giordano L, Romagnoli C (2019) The new model of continuous glucose monitoring sensor" ENLITE" in preterm infants.
- Harris DL, Battin MR, Weston PJ, Harding JE (2010) Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J Pediatr 157:198-202.
- Nally LM, Bondy N, Doiev J, Buckingham B, Wilson DM (2019) A Feasibility Study to Detect Neonatal Hypoglycemia in Infants of Diabetic Mothers Using Real-Time Continuous Glucose Monitoring. Diabetes technol Therapeutics 21: 170-176.
- McKinlay CJ, Alsweiler JM, Ansell JM, Anstice NS, Geoffrey CJ,et al. (2015) Neonatal glycemia and neurodevelopmental outcomes at 2 years. New England Journal of Medicine. 373:1507-1518.
- Galderisi A, Zammataro L, Losiouk E, Lanzola G, Kraemer K, et al., (2019) Continuous Glucose Monitoring Linked to an Artificial Intelligence Risk Index: Early Footprints of Intraventricular Hemorrhage in Preterm Neonates. Diabetes Technol Ther 21:146-153.